Deep TMS Therapy
The most advanced TMS therapy for treating depression, anxiety, and other conditions
What is deep TMS Therapy?
Deep Transcranial Magnetic Stimulation (TMS) therapy is a cutting-edge, non-medication treatment that is FDA approved for depression, anxiety, OCD and smoking addiction.
TMS uses a pulsating magnetic field designed to stimulate nerve cells (neurons) in the specific brain regions that are underactive in people with depression, anxiety, and other neuropsychiatric disorders. Over time, this “exercising” of those nerve cells strengthens them and their connections. Deep TMS is the most advanced form of FDA-approved TMS therapy as it stimulates more of the targeted brain areas (learn more about standard TMS versus Deep TMS).
Kadima is the most experienced provider of TMS and Deep TMS therapy in San Diego. At Kadima, all TMS therapy is overseen by Dr. David Feifel, a TMS pioneer who was the first physician to provide FDA-approved TMS in San Diego (2009). Contact Kadima to learn more about this revolutionary therapy and its potential benefits for you.
Did you know that most insurance providers in California now cover TMS therapy?
What is DEEP TMS Therapy?
Transcranial Magnetic Stimulation (TMS) is a revolutionary, drug-free, approach to treating depression, anxiety, and other psychiatric conditions.
Deep TMS is the most advanced type of TMS.
Deep TMS treatment involves 30-40 outpatient sessions (18-36minutes each, depending on your condition) over a course of 6 weeks.
Deep TMS is:
● Safe – No long-term side effects.
● Non-invasive, meaning that it does not involve surgery.
● Does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
● Non-systemic, meaning that it is not taken by mouth and does not circulate in the bloodstream throughout the body.
● FDA cleared for people suffering from major depression, anxiety (with Depression), OCD and smoking cessation, who do not benefit sufficiently from medication treatment..
“In contrast to the 20th century technology represented by conventional antidepressants, TMS is psychiatric treatment for the 21st century”- Dr. David Feifel
How TMS Therapy WORKS?
The principle behind TMS is the discovery that magnetic pulses focused on a brain region can be used to stimulate brain cells in that brain region. TMS applied over time at high frequency stimulates brain activity, whereas TMS applied over time at low frequency reduces brain activity. Since the symptoms of every psychiatric and neurological condition are associated with a unique pattern of abnormal activity (too high or too low) in certain brain regions, TMS aimed at those abnormal function areas can be used to alter their activity over time toward a more normal and healthy level.
TMS for depression treatment
Major depressive disorder is the most common condition that TMS is used to treat. Activity in the left frontal area of the brain, in a region called the dorsolateral prefrontal cortex, is reduced in patients with depression. High frequency TMS aimed at the left prefrontal cortex is used to return normal activity to that part of the brain and thereby eliminate or reduce the symptoms of depression.
TMS is a safe treatment option
The safety and efficacy of TMS for treating depression has been established in several well controlled research studies including a study sponsored by the National Institute of Mental Health. Based on this body of scientific evidence, in 2008 the FDA cleared TMS as treatment for patients with depression who have not benefited from antidepressant medication. Since 2008 over 18,000 patients have undergone TMS treatment.
“TMS is analogous to physical therapy, except a specific part of the brain is being ‘rehabed’ instead of an injured muscle and in place of a physical therapist, a pulsating magnetic field “exercises” the targeted brain neurons, strengthening the connections between them” –Dr. David Feifel
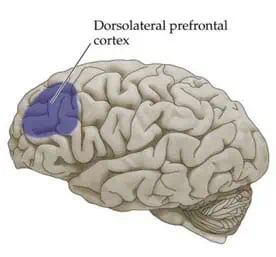
The dorsolateral prefrontal cortex on the left hemisphere is stimulated when treating depression and anxiety with Deep TMS.
“I understood that TMS had the potential to revolutionize the treatment of psychiatric conditions and so, as soon as it was cleared by the FDA in 2008, I worked to bring this technology to UCSD in the process I become the first provider of TMS therapy in San Diego and one of the first in the entire country. – Dr. David Feifel
Benefits of deep TMS THerapy
Effective Treatment
An effective treatment for severe depression and other mental health disorders.
Non-Invasive, drug-free
Deep TMS does not involve surgery, needles or pills.
Safe Treatment
TMS is a safe treatment with no long term side effects. You can go on with your day after the treatment
Not all TMS Devices Are Equal
TMS Devices
Currently eight TMS systems are approved by the FDA for treating patients with depression who have not benefitted from antidepressant medication or who cannot tolerate them. The first system, Neurostar, is based on the standard “figure-8” coil design. Dr. Feifel was the first provider in San Diego to offer Neurostar TMS therapy starting in 2009.
Three other TMS devices have more recently cleared by the FDA that use the figure-8 coil, Magventure, Magvita and Neurosoft. The limitation of standard coils designs such as the figure-8 coils is that they are only safely able to activate brain cells at up to 1-2 cm below the brain surface. The ability to activate brain circuits deeper than this without using unsafe and uncomfortable settings was a dilemma for the TMS field for years until a new coil design was invented.
Brainsway deep TMS
In 2013 the FDA cleared Brainsway TMS system for the treatment of Depression. This system uses a revolutionary coil called the ‘H’ coil, invented at the National Institute of Health (NIH). The H-coil is able to directly stimulate deeper into the brain than the any of the TMS devices that use the figure-8 coil (Neurostar, Magventure, Magvita, Neurosoft) allowing it to reach ‘limbic’ areas of the brain that regulate mood and motivation.
The H-Coil
The H-coil is integrated into a ‘helmet’ that sits over patient’s scalp. A session of “Deep TMS” or “dTMS” the name given to TMS treatment using the H-coil is typically 20 minutes, which is about half the duration of a typical Neurostar (figure-8 coil) TMS session.
Brainsway Deep TMS is considered the most advanced TMS system currently available. In addition to FDA clearance for Major Depressive Disorder, Anxiety associated with Depression, OCD and Smoking Cessation, the Brainsway system is currently approved (has CE mark) for several neuropsychiatric conditions in the European Union including:

KADIMA’s Deep TMS device. Each helmet contains a different FDA cleared coil is FDA designed to treat different symptoms by targeting different brain regions. From right to left: H1 coil major depression and Anxiety; H7 coil for OCD; H4 coil for smoking addiction
- Autism
- Major Depressive Disorder
- Bipolar Disorder
- Schizophrenia
- Post-Traumatic Stress Disorder
- Parkinson’s Disease
- Chronic Pain
- Multiple Sclerosis
- Stroke
- Obsessive Compulsive Disorder
- Smoking Cessation
Frequently Asked Questions
Most frequently asked questions and answers
A course of Deep TMS for depression consists of 30 treatment sessions, typically 5 days/week x 6 weeks, followed by a tapering-down period of 6 more sessions over an additional 3 weeks.
For OCD, the treatment course consists of a Preparation session followed by 29 TMS treatments, which are given 5 days/week x 5 weeks, and then 4 days/week in the final week.
For smoking addiction, the treatment course consists of a total of 18 TMS treatments given over 6 weeks, plus brief counseling at the end of each session. The TMS treatment sessions are given 5 days/week in the first 3 weeks, and then 1 day/week over the last 3 weeks.
Each Depression TMS treatment session typically takes 20 minutes using the standard treatment protocol. In 2021, the FDA also approved a shorter TMS protocol called Theta Burst, which takes only 3 minutes.
Each OCD TMS treatment session typically takes 30-40 minutes.
Each Smoking Addiction TMS treatment session typically takes 25-30 minutes.
The effect of TMS can take about 2 weeks for small changes to occur and usually begins after week 4
Insurance can possibly cover TMS, if you meet the authorization requirements outlined by your insurance company.
Repetitive TMS is a noninvasive form of brain stimulation used for depression. Unlike vagus nerve stimulation or deep brain stimulation, rTMS does not require surgery or implantation of electrodes. And, unlike electroconvulsive therapy (ECT), rTMS doesn’t cause seizures or require sedation with anesthesia.
Most people will qualify for Deep TMS. Please contact us and we can assess if TMS will be beneficial for you.
If TMS treatment is covered by insurance, the patient is responsible for any deductible, copay, or coinsurance outlined by the insurance company.
Cash pay for MDD TMS is $9200 and OCD TMS is $7500.
This treatment is:
- Safe – No long-term side effects.
- Non-invasive, meaning that it does not involve surgery.
- Does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
- Non-systemic, meaning nothing circulates in the bloodstream throughout the body.
- FDA cleared for people suffering from Major Depression who do not benefit sufficiently from medication treatment.
- A potential treatment option for many psychiatric and neurologic conditions.
Listen To deep TMS Therapy Reviews of Kadima's Patients
TMS is Covered By Most Insurances










Are you Interested in Deep TMS Therapy?
Want to check if your insurance covers for it?
(858)412-4130
Address: 3252 Holiday Ct Ste 112, La Jolla, CA 92037
Leave your details and we will contact you: